Biosynthesis of ornithine-containing lipids, a widespread class of bioactive lipids in eubacterial membranes
Principal investigator: Otto Geiger
Under phosphate-limiting conditions of growth, some eubacteria replace their membrane phospholipids by lipids not containing any phosphorus. In Sinorhizobium meliloti these phosphorus-free lipids are sulfoquinovosyl diacylglycerol, ornithine-containing lipids (OL), and diacylglyceryl trimethylhomoserine ( Fig. 2 ). Although OL are absent in eukaryotes and archaebacteria, they are widespread among eubacteria and stimulate the mammalian immune system. So far neither the biosynthesis of OL nor any genes or enzymes involved were known.
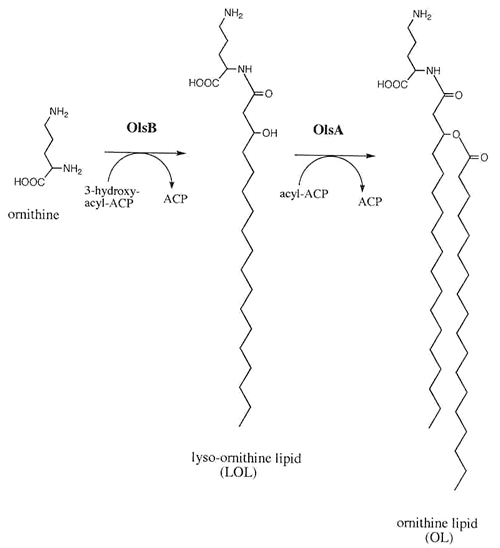
We have isolated mutants of S. meliloti deficient in OL biosynthesis and complementation of such mutants lead to the identification of two genes ( olsA and olsB ) required for OL biosynthesis. OL-deficient mutants of S. meliloti disrupted either in olsA or olsB show wild type-like growth behaviour and are capable of inducing nitrogen-fixing nodules on the sinorhizobial host plant alfalfa. OlsA shows homology to O -acyltransferases while OlsB matches the superfamily fold of acyl-CoA N -acyltransferases. Amplification of the genes and their expression in the corresponding OL-deficient mutant demonstrated that both are required for OL biosynthesis. Expression of the gene olsB is essential for the specific incorporation of radiolabeled ornithine into OL and overexpression of OlsB in an olsA -deficient mutant of S. meliloti leads to the transient accumulation of the presumed biosynthetic intermediate lyso-ornithine lipid. Overexpression of OlsB in Escherichia coli is sufficient to cause the in vivo formation of lyso-ornithine lipid in this organism and is the cause for a 3-hydroxyacyl-AcpP-dependent acyltransferase activity forming lyso-ornithine lipid from ornithine. These results demonstrate that OlsB is required for the first step of OL biosynthesis, in which ornithine is N -acylated with a 3-hydroxy-fatty acyl residue in order to obtain lyso-ornithine lipid. In a second step, lyso-ornithine-lipid is converted to OL by an olsA -dependent O -acyltransferase activity that requires acyl-AcpP as the acyl donor ( Fig. 3 ). OL formation in a wild type S. meliloti is increased upon growth under phosphate-limiting conditions. Expression of OlsB from a broad host range vector leads to the constitutive formation of relatively high amounts of OL (12-14% of total membrane lipids) independently of whether strains are grown in the presence of low or high concentrations of phosphate, suggesting that in S. meliloti the formation of OlsB is usually limiting for the amount of OL formed in this organism. ORFs homologous to OlsA and OlsB were identified in many eubacteria and although in S. meliloti the olsB and olsA gene are 14 kb apart, in numerous other bacteria they form an operon.
Presently, we try to understand which modifications of OL are occurring in different bacteria ( Rhizobium tropici and Burholderia cenocepacia ) and which functional consequences are associated with such changes. For example, we would expect that modified OL display altered bioactivity when applied to human macrophages or dendritic cells..

|
|
Fig. 2. Structures of phosphorus-free membrane lipids sulphoquinovosyldiacylglycerol, ornithine-containing lipid, and diacylglyceryl N,N,N -trimethylhomoserine in Sinorhizobium meliloti (taken from Geiger et al . 1999. Mol. Microbiol. 32:63-73).
|
|
|
Fig. 3. Model for the biosynthesis of ornithine-containing lipids (OL) in bacteria (taken from de Gao et al . 2004. Mol. Microbiol. 53:1757-1770).
|